How Can We Help?
I live with an ileostomy
About your ileostomy
Does it smell?
No. Providing the bag you are wearing has a good seal around the stoma and to your skin, the stoma or bag contents will not smell. If you can smell them then it is likely the seal is compromised and you may start to leak. Consider changing the bag.
Can I feel the stoma working?
In the early days you may as you get used to living with a stoma but it won’t be painful and after a while that will likely settle and you will not feel the stoma working or the bag filling. Often the only time you know the bag is filling is if you can feel the weight on your abdomen or it starts to bulge under your clothing.
The stoma is really red, is it sore?
No. A rich red/pink colour is a good sign of a healthy blood supply to the stoma/bowel. The bowel usually enjoys a rich blood supply so you should be more alert and seek medical advice if the colour changes or it starts to go black.
Should there be blood on the wipe when I clean the stoma?
Given the rich blood supply to the bowel it is normal to see some blood on the wipe when you are cleaning the stoma. If there is a lot of blood or blood is coming from inside the stoma, you should seek medical advice from your stoma care nurse, GP or surgeon.
Why is my stoma moving?
Even though the bowel is on the outside of the body the normal bowel action, known as peristalsis, will continue. This action moves food through the bowel and the stoma may appear longer and then shorter depending upon the bowel action.
Why is the stoma wet?
The bowel is a mucous membrane and it naturally has a mucous coating. When cleaning the stoma, the stoma itself will never dry, however you should ensure the skin around the stoma is clean and dry before placing a new bag onto your skin.
Can I bath/shower without a bag on?
There is nothing to stop you bathing or showering without a bag on although you may want to wear a bag in the bath in case the stoma works. In the shower, if the stoma works, it will flush away with the waste water. Avoid creams and lotions around the skin where the stoma is so that you don’t have any issues with the bag sticking once you are dry and putting on your new bag.
Can I go swimming with a stoma?
Absolutely. Swimming in the local pool or in the sea is perfectly fine. Your bag will not come off when it gets wet and will often adhere more tightly to the abdomen to prevent it coming off. Drainable bags, typically work with an ileostomy, are designed to stick strongly for at least a few days and stick more strongly when they get wet. If you are due to change the bag you may want to change it before you get in to the water given the adhesive bond is stronger.
What are the little nodules around my stoma?
These sound like granuloma. Little lumps or nodules around the stoma which can occur where your healthy skin is breaking down then healing due to constant exposure to faecal waste (poo). You may also notice that they bleed quite a bit when you clean the stoma. They can be difficult to get a bag over the top of when changing but try and cover them when you apply your bag to stop the skin from breaking down so that they start to heal and reduce in size. If they become troublesome or you are concerned please speak with your stoma care nurse who can guide you.
Why does it smell when I have been to the toilet?
Most people leave the typical toilet odour after emptying their bowels, however the contents of an ileostomy bag can seem more odorous. Bowel output for someone with an ileostomy still contains digestive enzymes which would normally be absorbed by the colon (which is no longer connected if you have an ileostomy). These enzymes break down the food that we eat and create odour. Whilst there are odour control products on prescription you may struggle to get these prescribed due to cost. Many supermarket air fresheners can help to mask toilet odour so shop around until you find something that works for you.
Diet
What can I eat?
Diet is a very individual choice for everyone, with or without a stoma. IA strongly encourages you to follow a healthy eating regime to ensure your body receives the right balance of nutrients and vitamins that it needs. This is especially important during your recovery period where the body would use more calories as part of the healing process. Alternatively, if you have been experiencing chronic illness, such as inflammatory bowel disease, you may have been on a restrictive diet to help manage your condition. It may no longer be necessary for you to follow a restricted diet once you have undergone surgery.
You should always follow guidance from your nurse or GP however in the main, most may follow a low fibre diet (less fibre/bulky fruit and vegetables) post-surgery for a few weeks to allow the bowel to settle and then gradually introduce more foods as time progresses.
Some tips to help with digestion include chewing food well before swallowing as well as avoiding eating and drinking at the same time as it can introduce more air into the digestive tract. As the bowel settles you may notice that air is much of a problem.
Can I eat fruit and vegetables?
You may have read or be unsure about whether you can eat fruit and vegetables with a stoma. Absolutely you can and they form an important source of vitamins and nutrients in your diet. Some may be nervous in case they get stuck because of their bulk and avoid them. We would encourage you to try all foods unless you have a medical or religious reason for doing so. Introduce new foods gradually into your diet so you can see how your body processes them. Avoid too much at once, little and often is key until you build up your confidence with your diet. Also ensure you chew your food well to help digestion.
Remember you can always puree or mash foods such as fruit and vegetables to gain the nutritional benefit from them if you are concerned about eating them. Some prefer to cook their fruit and vegetables well before eating them, some avoid raw fruit and vegetables but many eat them as they wish without any problems.
Can food block my bowel or cause an obstruction?
You may have read on the internet how people avoid certain foods for fear of bowel obstruction. Foods such as nuts, sweetcorn, peas, celery and popcorn amongst other things. It is true that in certain people there is a slight increased risk of bowel obstruction but there may be other factors that increase that risk. For that reason, IA strongly encourages you to try foods for yourself, little and often, so you can see how your body digests them. Chew them thoroughly.
Do different foods affect my stoma output?
Because diet is so individual, what may loosen one person’s stoma output may not affect another. It’s always best to try them and see first rather than avoid anything and miss out on it for no good reason. In general, based on people’s experiences rather than any research, foods such as banana, rice pudding, jelly, marshmallows, peanut butter and apple sauce tend to thicken stoma output as they require the bowel to work more and therefore slowing it a little encourages more liquid absorption.
Again, based on experience and certainly not a definitive list – leafy green vegetables, caffeine products and alcohol may stimulate the bowel and either work through more quickly or create more gas in the bowel.
Given our individuality it is important to see what affect these foods have on you rather than avoiding them because they have affected others. Little and often is key with new foods until you know how your body processes it.
Ileostomy surgery
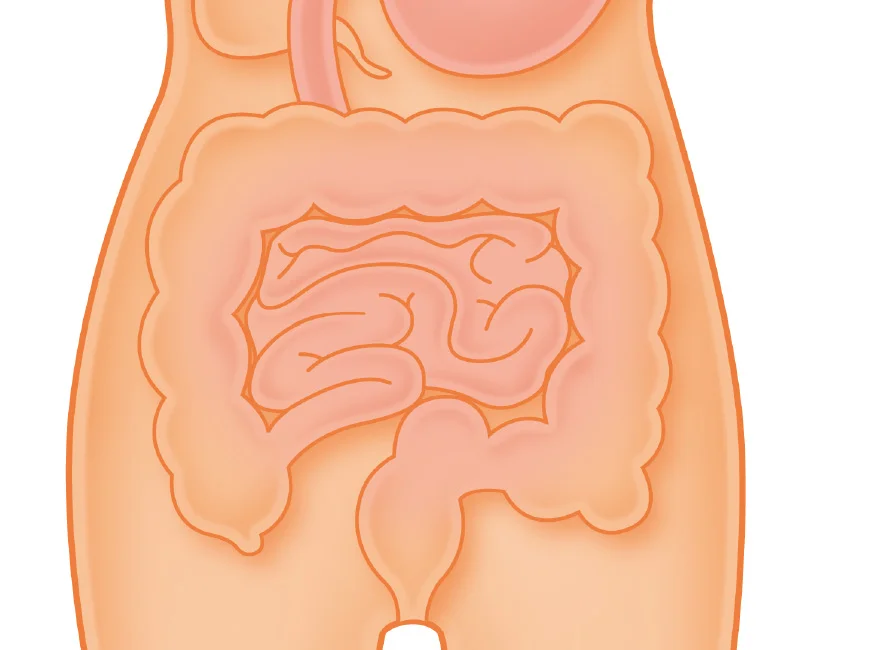
What is an ileostomy?
An ileostomy is formed from the end of your small intestine (ileum). The end of the small intestine is brought out to the surface of the abdomen and stitched to the skin. A stoma bag is worn over the stoma to collect faecal (poo) waste. The ileostomy is reddish pink in colour (similar to the inside of your mouth) and is often the size of a strawberry. Depending upon your surgery, your surgeon will try and make the stoma at least an inch or two long so that it droops down in towards the bag. Therefore, as the stoma works the waste drops into the bag and away from your healthy skin.
Where will the ileostomy be on my tummy?
Placement of the stoma is often down to whether surgery is planned or emergency, whether you have had previous abdominal surgery, surgical ability and lifestyle choices.
An ileostomy is usually placed to the patient’s right side of the abdomen (halfway between the belly button and top of the pubic area) although this will not always be the case if other factors prevent this. Prior to planned surgery, a stoma care nurse will assess you and discuss lifestyle preferences so they can ‘site’ your stoma. They will take into account body shape, ability and lifestyle choices, e.g. clothing choices.
Sitting a stoma involves marking an ‘X’ on your abdomen so the surgeon knows where to place the stoma in surgery; although this is not guaranteed as the surgeon may have to change position of the stoma based on what he/she finds internally when they operate. Siting is much more difficult during emergency surgery given it is an emergency situation and therefore the surgeon will do their best to place the ileostomy in a ‘suitable’ location, however the priority is to preserve life.
Why do I need an ileostomy?
An ileostomy can be formed for many reasons; chronic (long term) or acute (short term) illness, bowel motility issues (how well food moves through the bowel), trauma to the abdomen, bowel ischaemia (blood supply issues) or perhaps difficulty during childbirth. Illness such as ulcerative colitis or Crohn’s disease (known as Inflammatory Bowel Disease (IBD), cancer, diverticulitis/diverticular disease or polyposis (also known as FAP) are common reasons for an ileostomy being formed.
I don’t want an ileostomy?
The decision for surgery is always a difficult one. It is often done to preserve life or improve quality of life and is not usually the first choice unless you are in an emergency situation. Gastroenterologists (doctors who specialise in the digestive system) may suggest surgery where you are being treated for disease and alternative options are not working or not available.
It is important that if the option of surgery is raised by your gastro team or surgeon that you have full, open discussion with them so you can understand what the surgical option means for you and they can understand the concerns that you have.
How can I make a decision?
That’s a difficult one. Nobody can tell you what to do, however you should talk it through with your surgeon, stoma care nurse, GP or family member/friend. A surgeon will not suggest surgery if they do not think that it will have a positive effect on your health or wellbeing. Surgery is usually suggested where medication has not been successful or if the situation is an emergency and they need to preserve life. Perhaps create a list of the good and bad points as you see it. What is life like before surgery and what do you think it will be like after surgery? Remember an IA trained volunteer who is living with a stoma or pouch can often help you with any questions about what it is generally like for them living with a stoma. Never feel you are alone in this.
Is there an alternative to living with an ileostomy?
There are alternative options but these options are not without their own difficulties and are not necessarily suited to everybody. It is best to discuss your concerns with your surgeon or gastro team and what, if any, alternatives are available to you.
How is ileostomy surgery performed?
Surgery can either be done as ‘open’ surgery (laparotomy) where the surgeon will make a longer incision down the abdomen to access your bowel or if your surgeon is trained in ‘keyhole’ surgery (laparoscopic), a series of small holes (known as ports) are made in the abdomen for the surgical instruments to be inserted.
Should I have open or keyhole surgery?
This will often be down to your surgeon and will depend upon your own medical situation and whether the surgeon performing the surgery is trained in keyhole surgery. Not all surgery can be done through keyhole surgery so you should discuss your preferences with your surgeon before surgery is performed. Keyhole surgery does not involve cutting muscle layers in the abdomen and therefore recovery is usually much quicker than ‘open’ surgery. Scarring from keyhole surgery is much less and will usually fade over time. Sometimes, even if keyhole surgery is planned, it may be necessary for the surgeon to revert to open surgery part way through although they should discuss this with you ahead of surgery commencing so you are aware.
Products and Supplies
Where do I get stoma bags from?
Your stoma care nurse will cover everything with you either before, or soon after, surgery so that you have all the supplies you need. Stoma bags are only available on prescription in the UK and your nurse will ensure your GP has all the information required to fulfil your prescription requirements when you first get home from hospital. Whilst in hospital, your nurse and ward staff will ensure that you have the relevant products available to you and discharge you with sufficient supplies until your first prescription is received. Prescriptions can be fulfilled either by your local pharmacy or a delivery appliance company – which company and which method is used is entirely your choice.
If your GP surgery offers electronic prescriptions (EPS), you can order your prescription via the GP and then nominate who should supply them.
What types of bags are there?
There are lots of different bags from many product manufacturers. For example bags can be one piece or two piece; they can be drainable or closed; they can be covered or transparent; they may have a filter or no filter; they may have a different type of closure. Be guided by your stoma care nurse initially and let them advise you as to the bag that they think is best for your clinical needs. They will cover all this with you when you meet them usually before surgery, or after if it is an emergency.
How many bags do I need?
Bags are usually in boxes of 10 or 30 depending upon the style you use. Depending upon clinical need you should only order the number required. Your GP will monitor your prescription request and authorise it based on what they have been advised by the stoma care nurse is required. If you need to change the prescription or increase the amount being ordered please speak with your stoma care nurse or GP so they are happy with the change. If you order a different product or change quantities that may result in the GP rejecting or querying the prescription and lead to delays. We strongly advise against stockpiling bags to ensure you have sufficient, to avoid unnecessary waste. We know from experience that sometimes stockpiling your bags when a product change is recommended means the old style of bag is wasted at great cost to the NHS.
What if I don’t know what products I need?
Your stoma care nurse will assess your requirements and arrange for you to receive the products you need to look after your stoma. Deciding which products you need can be overwhelming alongside coping with a new stoma so we strongly recommend you are guided by your nurse until your confidence develops and you learn about the different products available.
Can I get any product I need to look after my stoma?
Product accessibility is always based on clinical need. If you don’t need a particular product that you know someone else is using it is likely because you don’t need it. Your stoma care nurse or GP will assess your needs based on when you were discharged from hospital or if you are experiencing any problems that require their intervention. Quite simply, the cost of stoma care products to the NHS is vast and therefore everyone, stoma or not, plays an important role in reducing costs to the NHS and only using the products that they require.
If we all, stoma or not, abuse that, then costs will increase and services will undoubtedly start to be affected. However, if you are experiencing problems with your stoma please discuss this with your stoma care nurse or GP rather than avoiding doing so to avoid the cost. The problem may be easily solved for little cost but may become bigger and costlier if it is left.
If you want to consider changing your stoma bags to a different or more suitable product please speak with your GP or stoma care nurse first. IA strongly recommends you try a sample of the product first (usually available through the product manufacturer online) to ensure it is suitable and meets your clinical needs before ordering a box.
Do I have to pay for my prescriptions?
If your prescribing service (GP, hospital or other local service) is based in England, and you normally pay for your prescriptions, you may have to pay the current rate for each item on your prescription. This will depend upon whether you are entitled to medical exemption. Exemption will usually be because you have a permanent stoma and/or other condition that entitles you to free prescriptions and therefore once you have applied for and receive a ‘medical exemption certificate’ you will not have to pay for any prescription (stoma related or otherwise). If your stoma is temporary, you should discuss this with your GP, however you are not automatically entitled to free prescriptions. You should not indicate on your prescription that you have medical exemption (and avoid paying) unless you physically hold a medical exemption certificate, and it is in date. Failing to do so may result in costs being reclaimed and a penalty fine. More information is available here.
If you are not entitled to medical exemption and you are receiving multiple items on prescription each month consider a pre-payment certificate to reduce your costs.
If your prescribing service/GP is based in Scotland, Wales or Northern Ireland you do not pay for your prescriptions however you must follow their local guidelines when requesting items from your GP on prescription.
If you are based in the south of Ireland you should follow local guidelines when ordering, paying for and collecting your prescriptions. These are not normally free unless you qualify.
Recovery and return to Work
What happens after surgery?
Where possible, you will be encouraged to get out of bed and either into a chair or taking a few steps, often from the following day, so that the body is moving. Movement can also help the stoma to start working. It is important that the surgical team see the stoma working so that you can start to eat and drink again. Although this will be a cautious step at first, it is so they can see that what is going in, is also coming out through the stoma. You will be encouraged to eat a soft diet at first. Foods such as soup, rice pudding, yoghurt, porridge, semolina and mashed potato amongst other things will likely be on the menu for the first few days until the bowel is working. If you follow any particular dietary regime (for health reasons or otherwise) discuss this with your local nursing/catering team so that the right foods are available to you. Once the team see the stoma working, you will be encouraged to broaden your food intake to help your recovery.
How long will I be in hospital?
Length of hospital stay will depend upon many factors: your reason for surgery, the surgery itself and your recovery are many factors. One of the most important things is ensuring that your stoma is working after surgery, you are eating and drinking (albeit perhaps lightly) and you can care for the stoma yourself or have someone who can assist you in your home setting. Your stoma care nurse will ensure that they are satisfied that you can do this before you will be discharged.
Some may be discharged from hospital after 3 or 4 days with others in hospital for longer, depending upon the above and their personal circumstances. Discuss this with your surgeon or stoma care nurse before surgery although it is a guide and you should prepare for a longer stay, if it is necessary.
How quickly will I recover?
Recovery is very individual and you must be guided by the advice given to you on discharge. There is no ‘hard and fast’ rule that says you must be able to do something within so many days or weeks of surgery. Once home, setting yourself realistic goals can help you to move forward. Where you are able to, and you feel confident, perhaps walking in the garden or maybe to the first lamppost in your street may be a good starting position. Where possible, ask someone to walk with you so they can help you if necessary. Sometimes you may feel ready but become tired very quickly so you should not overdo it too quickly. Remember that walking to say the fifth lamppost after a few days may seem easy but you also have to walk back again.
Listen to your body. If it feels OK, it probably is, but if it doesn’t then stop or slow things down. Perhaps you are moving forward too quickly and need a bit longer.
When can I start driving again?
You should not start driving again until your surgeon or stoma care nurse has advised that it is OK to do so. If you do, and you have an accident, it may invalidate your vehicle insurance. If in any doubt, check with your surgical team or stoma care nurse AND your insurance company before you start driving again. Your insurance company will likely be guided by what your surgeon or stoma care nurse says. Be aware that you MUST continue to wear a seatbelt in a car, even if you think it may strap across your stoma bag or surgical scars. There is no medical exemption and you can often carefully position the belt so that it is comfortable and you remain safe and within the law.
When can I lift things?
One of the biggest tendencies after abdominal surgery is feeling well and starting to lift heavy things. You should listen to the guidance given to you and not lift anything heavier than a kettle for the first 6 weeks to avoid straining yourself and causing internal damage. This may differ according to the type of surgery you have had (keyhole or open surgery) so discuss this before you are discharged.
When can I return to work?
This is very individual depending upon your surgery, recovery and what type of job you do. If you have had keyhole surgery you will likely be able to return to work more quickly than open surgery allows, given the healing process is different. As an indication you are likely to be off anywhere from a few weeks after keyhole surgery and say 6-8 weeks after open surgery, however be prepared for this to be longer. You should not however return to work until your medical team has advised you that is it safe and declared you ‘fit’ to do so.
Often people will agree a return to work schedule with their employer or occupation health team, if you have one. This may be reduced hours for the first few weeks, perhaps working from home if your job allows or a temporary/ possibly permanent change to your work duties. Returning to work too quickly and doing long hours will potentially have an impact on you physically so again listen to your body and take it slowly. Remember, travelling a distance to get to work can be as tiring after surgery as the work itself.
If your job is a manual one, you may need to agree lighter duties with your employer for a period of time whilst you settle back to work and minimise any risk to your recovery. Sometimes this can be a permanent change but discuss this with your surgical team, stoma care nurse and employer/occupational health team when the time is right. You should never be pressured into returning to work too early or to perform a role that you may feel disadvantaged in living with a stoma, so if necessary, speak to your local Citizen’s Advice about your situation and your rights.
Am I disabled?
Disability is not about whether you can walk a certain distance or have fully functioning limbs. Whether or not you feel disabled or class yourself as disabled, in the eyes of the law living with a stoma does class you as having a disability. You are therefore covered under the disability provisions of the Equality Act 2010. The act is there to protect people living with long term chronic illness or a prescribed disability. It does not mean you would be entitled to everything that someone else who is classed as having a disability would. Disability is as much about ability and how your ability is assessed.
Am I entitled to a blue badge?
A stoma itself does not automatically qualify someone for a blue badge so that they can legally use a disabled parking space or park where such a badge would allow. If you have additional health issues or your personal circumstances dictate, you may qualify under one of these conditions, however a stoma alone would not normally entitle you to a blue badge.
Will I be treated any differently at work because I have a stoma?
Disability legislation within the Equality Act 2010 says that you must not be unfairly disadvantaged because you have a stoma and that you must be afforded the same opportunities as anyone not covered under the Act, even if this means that the employer is responsible for making reasonable changes or concessions in the workplace.
For example, if your time away from the desk is monitored, say you work in a call centre, this may mean that your employer will need to allow you longer away from the desk for a toilet visit or at ad-hoc times as is necessary. This is because you may need longer to empty your bag (whilst your confidence builds), or perhaps your bag needs emptying more during the day.
Alternatively, if your toilets do not have bins in but there is a chance that you need to change your bag at work, the employer must install bins in to the toilets so you have somewhere appropriate to dispose of it not necessarily sanitary bins however, unless your employer falls within the prescribed guidelines. You can always contact IA for guidance both for yourself and for your employer, if required.
What if I need time off for medical appointments?
Discuss this with your employer. Most will be reasonable and allow the time required, however there may be circumstances when it becomes difficult for the employer to remain operational and they may refuse. Usually you can agree something that works for all parties, however your employer is not allowed to refuse you on the basis that others in the team need less time for medical appointments.
Relationships and Pregnancy
Will having a stoma make it difficult with my existing partner?
Understandably you may be concerned about having surgery whether or not you are in a relationship. Some are concerned how their partner will react when they see the bag. Will they be supportive? Will it lead to rejection? Will they still love or fancy me?
Your health must come first and if you need surgery please do not delay it because of relationship concerns. Beauty is more than skin deep and a bag can be seen as a sign of strength and courage.
If you are in a relationship and need surgery, discuss this with your partner so they understand what you are going through. Let them alleviate any concerns you may have and reassure you of their commitment. Often, if they have seen you experience ill health, they may find some comfort in knowing that a stoma is going to provide relief to your symptoms. Many partners are unsure of what to say or how to react for fear of upsetting you and may need to take a lead from you once they understand how you feel. It may also help them to tailor their support during and after surgery.
Read IA’s leaflet for families and carers.
Will I find a partner if I have a stoma?
Yes you will. Sadly not everyone truly understands what a stoma is and they may feel uncomfortable about forming a relationship, either short or longer term. As the cliché goes – there’s plenty more fish in the sea! If someone cannot accept you for who you are, you need to value yourself more and let them move on. Beauty is much more than how we look.
Understandably meeting someone new may make you feel nervous about telling them of your past surgery and you won’t truly know whether they will understand or not until you tell them. Perhaps as you get to know that person you can gently introduce the subject of your past health and explain to them how you felt at the time, what your experience was like and the difficulty you faced. From this you can gauge their understanding, how empathetic they may be or their level of compassion before you tell them about your stoma.
Can I have sex with a stoma?
Absolutely. Ordinarily a stoma itself doesn’t mean that sex is not possible or should be any different from what you would normally enjoy. Depending upon your reason for surgery or any health condition, you may need to make some adjustments, but these can usually be worked through with your partner until a solution is found that works for both of you. It is important that you feel relaxed, wanted, valued and sexy during times of intimacy and having a stoma does not mean that that cannot be the norm.
Some men may experience erectile dysfunction, again depending on surgery, and if this lasts more than a few weeks once they feel ready after surgery, discuss this with your stoma care nurse, surgeon or GP. Sometimes internal bruising or swelling around nerves can lead to temporary issues with obtaining an erection.
Women may experience vaginal dryness or loss of sensitivity post-surgery and again you can discuss this with your stoma care nurse, surgeon or GP. These people are professionals and are there to help you however embarrassed or awkward you may feel.
Sometimes a little planning can help you relax before intimacy such as emptying/ changing your bag beforehand or wearing something that makes you feel good about yourself and covers your bag initially until you feel more confident.
Medical advice is that you should never introduce any object into your stoma unless it is under medical supervision.
Can I get pregnant with a stoma?
Yes. It is completely possible to conceive and experience a healthy pregnancy if you have a stoma. As your baby grows and your abdomen changes shape, you may need to adjust the hole cut in your stoma bags to cater for stoma shape. Please discuss any concerns you may have with your midwife and nursing team about your stoma and delivery. IA can always put you in touch with a trained volunteer who has been through pregnancy with a stoma if that helps. Please contact IA national office.
Stoma problems
What problems can I experience with a stoma?
Like any surgery, it is often not without risk. Problems that people may experience with a stoma are leakage, sore skin, hernia or bowel obstruction, however not everyone will experience any or all of these problems. If you are experiencing issues speak with your stoma care nurse who can give you specific advice and help you to correct or manage the situation.
My bag is on but the skin under the stoma bag is itching?
This is a sign that faecal waste (poo) has got under the bag stuck to your skin and is starting to leak. Normally this type of feeling is the waste starting to burn your skin. Consider changing the bag to prevent the skin becoming sore.
Why do I keep leaking?
Understandably leaks can knock your confidence and get you down. Leaks usually occur for a reason, however consider the following options and speak to your stoma care nurse for guidance:
- Are you cutting your stoma bags to the correct size? You should not let the cut bag overlap the stoma otherwise it won’t stick but you also need to cover as much healthy skin around your stoma as possible. If you are new to surgery please measure your stoma regularly and adjust the hole to be cut.
- Is your skin clean and dry before you place a new stoma bag onto your skin?
- Are you getting a good bond between your bag and your skin when you apply a new bag? Warming your bag before applying (under your arm or against your skin) and pressing down firmly after applying and holding your warm hands over the top of the bag for a few minutes can help the bag seal to your skin.
- Do you have any dips, creases or folds in the skin under your stoma bag that may prevent a good seal and your bag to leak? Speak with your stoma care nurse if you do for guidance.
- Are you leaving a little air in the bag after emptying the bag so the waste can drop down into the bag? If you notice waste collecting around the top of the bag and stoma, try and leave a little more air in the bag after emptying so the waste can drop down into the bag and away from the stoma.
- Have you noticed that your stoma is retracting (going back into the abdomen) or becoming flush to your body? Speak with your nurse if you think this is happening.
Why is my skin red and/or broken around my stoma?
Sore skin can be difficult to manage especially when you have to put a bag on it. Thankfully hydrocolloid (the ‘light beige’ colour material the flange on your bag is made from) is skin friendly and can help your skin to heal when it is stuck to your skin. Sore skin is usually caused by a leak or where the faecal waste is in contact with healthy skin and burns it. If you have a leak, change your bag before the skin breaks down. If the soreness is around the edge of your stoma, check you are cutting your bags to the correct size and covering as much healthy skin as possible. If you are new to surgery you may need to measure your stoma more frequently until it settles in size. Soreness can very occasionally occur if your skin reacts to the bag but this is rare. If the problem continues please speak to your stoma care nurse for guidance.
Why do I have a bulge around my scar or behind my stoma?
Lumps or bumps on the abdomen happen to anyone if our body shape changes or perhaps after abdominal surgery. Where you have had surgery (and sometimes not) we can develop a hernia in the abdomen where the muscle layer in the abdomen is weak (from surgery) and the bowel itself pokes through under the skin. Many people live with a hernia and ordinarily they are not a problem. The bulge may be more prominent when you are stood up but when you lay on your back it should disappear (as the bowel drops back in to the abdominal cavity). If the bowel does not drop back, becomes painful or you think it is stuck please seek medical guidance. In extreme cases the bowel can become trapped and this can be life threatening if it inhibits blood flow to the bowel.
What should I do if my stoma has stopped working?
In rare cases, if the stoma stops working, as in it has been many hours since the bowel last passed anything and you would normally expect it to have done so, think about any other feelings you may be experiencing. Are you feeling bloated (full but haven’t eaten anything for a while)? Are you experiencing any tummy pain? Are you feeling/have you been sick?
If the answer is yes to any of those, your bowel may be (partially) blocked. The important thing to do is not panic. Stop eating but it is important to keep drinking, consider sips. If the symptoms are mild, try moving around a little or laying on your side and very gently massaging your tummy. Sometimes a warm bath can help you to relax and get things moving again.
If symptoms persist, or become worse, or you are struggling to keep anything down, phone 111 for guidance or go to A&E.
Travel
Can you fly with a stoma?
Absolutely. Many go short distances at first to gain their confidence before long haul journeys, however in general, flying doesn’t impact someone with a stoma.
Will they need special travel insurance?
Travel policies for anyone with an underlying health condition can usually be purchased from most insurers. IA strongly recommends that all medical conditions, hospital stays and medical information is disclosed to ensure that the insurance is not invalidated in the event of a claim. Many insurers on the high street or online will be able to provide a quotation for travel insurance.
One of my travel companions has a stoma, what should I do?
Be led by your companion. If they are self-sufficient then there is nothing you need to do, they will manage everything as they do normally. Be aware that not everyone is as open about living with a stoma so they may feel self-conscious if they have not spoken to you about it much. If they are stopped by airport security as part of a body search or scan then please be sympathetic to them and give them the time that is required to satisfy security. Again if they have not mentioned it, perhaps it is best not to raise the subject unless they do.
If they are new to a stoma, it may take them a while to build their confidence being away from their usual surroundings or home cooking so please be patient if they need a little time to settle and build their confidence. You can have just as much fun!
Can I fly with a stoma?
Absolutely. Many go short distances at first to gain their confidence before long haul journeys, however in general, flying doesn’t impact someone with a stoma. Of course you may be more nervous about access to toilet facilities, your hand luggage or what you will eat/drink but they can be overcome with some up front planning. For example, if you can pre-book your seat, go for one close to a toilet. Food usually shouldn’t be an issue but speak to airline staff beforehand if you have any concerns or specific dietary preferences. With regards to access to your bags during the flight, pack some pre-cut bags, wipes, disposal bags and other accessories in your hand luggage in case you need to change your bag during the flight. Be aware, however, that you are not allowed to carry scissors in your hand luggage and any liquids, creams or pastes must fall within allowable limits otherwise you risk them being confiscated by security and taken away from you.
How many bags should I take on holiday with me?
IA strongly recommends you take at least twice the amount usually required for the period of time you are away. You may change your bag more frequently if you are in a hot climate, regularly in the water, perhaps an untimely bout of diarrhoea due to a change in diet or unforeseen delays leaving you short of bags. Remember you will be subject to hand luggage limits if you are flying so if you do pack your stoma bags in your suitcase which goes into the aircraft hold, consider spreading your supplies around multiple suitcases (if a few of you are travelling and that is an option) in case a suitcase goes astray. Alternatively pack a good supply of stoma bags in your hand luggage (again you will be subject to limits). You can however request an increase to your hand luggage allowance for medical purposes with a letter from your GP and airline agreement beforehand – please do not leave it until your departure to rely on a hand luggage allowance increase. This will not usually include items such as liquids, pastes or creams (e.g. accessory products) and these will usually need to go into your suitcase if over a certain size/volume.
Do I have to tell airport staff I have a stoma?
Not by law but you may wish to if you are going through an airport scanner or are subject to pat down. This will alert them to feeling or seeing the bag beforehand. You may be asked to swab the outside of your bag for illegal substances. This can usually be done by placing your hand under your top and swabbing across the bag without the need to display it before it is analysed by staff. Alternatively, if you are asked to display your bag or remove it (although the latter is rare) you are entitled to request a private room to do so. Thankfully most airports around the world are familiar with stoma bags and more sensitive to people’s feelings, however there is a chance that some staff may not be. If you find yourself in that situation, try and remain calm, request some privacy if you feel you need it and as difficult as it may be, politely answer their questions.
You can request a travel certificate from IA national office which explains what surgery you have had in multiple languages although it will not avoid you bypassing security or fulfilling their checks.
What if I get diarrhoea while I am away?
This can occur at any time, on holiday or at home, whether or not you have a stoma. It is important that you remain hydrated if you do have diarrhoea. Your body can manage without food for a while but it must remain hydrated for it to remain effective and for you to remain well. Assuming there is no medical reason not to, you can either take with you, or purchase locally, diarrhoea remedies to help manage your upset tummy. Additionally, a supply of re-hydration sachets such as ‘Dioralyte’ or suitable alternative will help you to stay hydrated. Water alone can have a more negative effect if you do have diarrhoea with an ileostomy as it will not replace lost electrolytes.
IA’s leaflet Staying Hydrated will help you to manage your hydration levels effectively. If concerned, always seek medical advice as soon as possible.
Should I try the local food and water?
Part of the experience of another country is their culture. Trying local dishes is part of that, however understandably you may be nervous given your stoma. Having a stoma doesn’t necessarily mean you will experience problems eating foods you are not used to; it can happen to anyone at any time. If you are cautious maybe try a small amount first for a few days until you build your confidence.
Drinking the local water however may cause an upset stomach, especially if it is a different balance in minerals than you are used to. Be guided by the advice from your travel company rep or whatever is available locally. If in doubt boil your water first and allow it to cool or purchase bottled water to drink and when brushing your teeth.
Where do I get travel insurance?
Travel insurance is widely available across the high street or online. Banks, post offices, supermarkets and travel agencies are among some of the places on the high street you may want to obtain information from. Alternatively, searching online will provide a range of insurers. Obviously whether they are the best for you, or the cheapest, will require some digging around. Most will likely cater for someone living with a medical condition and remember to declare everything to the insurance company when you obtain a quote so you are fully covered. If in doubt, tell them about the treatment or hospital stay to avoid an invalid insurance claim when you need it most. Please remember to declare any stay you have had in hospital or medical treatment you have received in addition to any conditions.
Some companies specialise in certain conditions so if you have, or have had, cancer in the past you may want to consider a specialist company who are more understanding. Given the individual nature of individuals and the wide range of insurers, we do not have a list but we do recommend you shop around to get the cover you require at the right cost. If you are looking for a specialist and are struggling, perhaps you could post a request on IA’s social media pages or IA’s forum to see if anyone else can offer any suggestions.
I am supporting someone with an ileostomy
About an ileostomy
Does it smell?
No. If there is a good seal between the bag and their skin, the stoma or bag will not smell.
The stoma looks really red, is it sore?
No. A rich red/pink colour is a good sign of a healthy blood supply to the stoma/bowel. The bowel usually enjoys a rich blood supply. If the person notices a change in colour to the stoma, encourage them to seek medical guidance.
Can you go swimming with a stoma?
Absolutely. Swimming in the local pool or in the sea is perfectly fine. The bag will not come off when it gets wet and will often stick more tightly to the abdomen to prevent it coming off.
Can you exercise with a stoma?
Exercise is very important for everybody, whether or not they have a stoma. Many are concerned that exercise may damage their stoma or cause additional problems. While there is little research in this area, people’s experiences show us that exercise is fine, once their surgeon or nurse has confirmed it is fine to do so after surgery. Cardio exercise such as walking, running, cycling or a cross trainer is usually a good starting position but this will depend on their level of fitness. Encourage them to speak with the stoma care nurse.
Will I need to help my partner/family member change their stoma bag?
Your stoma care nurse will identify if someone is able to change their bag on their own or requires assistance at home. They will not be discharged from hospital unless they can demonstrate self-care or arrangements are in place for someone to support them. If you are their carer then you should be invited in to walk through what is required to change the bag with the nurse so you have a good understanding and are able to demonstrate doing so.
If they receive external care, your nurse should ensure that there is provision in place for the caring team to help with changing the bag. Many who work in a care setting have experience of changing a stoma bag, however the nurse will ensure that training is available, if required.
Diet
What can you eat?
Diet is a very individual choice for everyone, with or without a stoma. IA strongly encourages anyone with a stoma to follow a healthy eating regime to ensure their body receives the right balance of nutrients and vitamins that it needs. This is especially important during their recovery period where the body would use more calories as part of the healing process. If someone has been experiencing chronic illness, such as inflammatory bowel disease, they may have been on a restrictive diet to help manage their condition. It may no longer be necessary for them to follow a restricted diet once they have undergone surgery.
They may be advised to follow a low fibre diet (less fibre/bulky fruit and vegetables) post-surgery for a few weeks to allow the bowel to settle and then gradually introduce more foods as time progresses.
Once the bowel has settled, encourage them to introduce new foods gradually so they can see how their body reacts and they can build their confidence with food. In the majority of cases everything will be fine and they can start to enjoy food again, especially if they have been restricted due to illness previously.
Some tips to help with digestion include chewing food well before swallowing, avoiding eating and drinking at the same time as it can introduce more air into the digestive tract and cooking raw vegetables well so they are slightly mushy when eaten.
Ileostomy surgery
What is an ileostomy?
An ileostomy is formed from the end of the small intestine (ileum). The end of the small intestine is brought out to the surface of the abdomen and stitched to the skin. A stoma bag is worn over the stoma to collect faecal (poo) waste. The ileostomy is reddish pink in colour (similar to the inside of your mouth) and is often the size of a strawberry. The stoma is ideally spouted (about 1-2 inches long).
Why do people need an ileostomy?
An ileostomy can be formed for many reasons; chronic (long term) or acute (short term) illness, bowel motility issues (how well food moves through the bowel), trauma to the abdomen, bowel ischaemia (blood supply issues) or perhaps difficulty during childbirth. Illness such as ulcerative colitis or Crohn’s disease (known as Inflammatory Bowel Disease (IBD), cancer, diverticulitis/diverticular disease or polyposis (also known as FAP) are common reasons for an ileostomy being formed.
Is there an alternative to living with an ileostomy?
There are alternative options but these options are not without their own difficulties and are not necessarily suited to everybody. It is best for the patient to discuss their concerns with their medical team so they can understand what options are available to them.
What can I do to support someone who has been told they need to have surgery?
Being told you need to have surgery can be a shock for anyone. It can generate feelings of fear, apprehension, anxiety, anger or denial. The person may withdraw into themselves whilst they think about what is happening to them or they may want to talk about it. Sometimes it can be difficult to know what is best and how to support someone. Perhaps just letting them know that you are there for them to listen if they’d like to talk or maybe to support them at any medical appointments, if that’s what they’d like. Given we are all different, some people may just like space whilst they come to terms with the need for surgery and will be ready to talk about it once they’ve understood it more.
I’ve seen someone suffering with illness and I’m glad they are having surgery, should I tell them?
Perhaps wait and see if they want to discuss it with you. If they do and they show signs of concern for your friendship/relationship once they have a stoma perhaps you can reassure them that you’ve seen how they struggle with illness and while having a stoma will be new, so many people get a new lease of life free from illness. They may initially just want reassurance that you will still see them as the same person and that you will be there to support them, if they need it.
What if I think surgery is the best option for someone but they are refusing to have the surgery?
You cannot force anyone to have surgery. It must be their decision, on their terms when they feel it is either the right time or the best option for them after discussion with their medical team. It can be difficult when you are on the outside looking in and can see the positive benefit it will bring but agreeing to surgery is a first step towards acceptance, even if someone really hates the idea. Be there for them if they want to talk and perhaps help them to make a list of all the good and bad points that they think it will be like before and after surgery.
What support is available to someone who is going through surgery?
Someone who has been told they need to have surgery will have the support of their medical team, be it gastroenterologist, surgeon or their stoma care nurse. A stoma care nurse is a specially trained nurse in stoma management who will be able to answer their questions and be there for them before, during and after surgery. Once someone has a stoma, a stoma care nurse at their local hospital will remain a point of contact if they experience any stoma-related problems, in addition to their GP.
Products and Supplies
Where do they get stoma bags from?
All stoma bags are prescribed by the GP. The stoma care nurse will ensure everything is set up with the GP before someone leaves hospital so they receive the stoma supplies that they need. Prescriptions can be supplied either by the local pharmacy or a delivery appliance company which the stoma care nurse can advise on.
What type of bags/products are there?
There are lots of different bags from many product manufacturers. For example, bags can be one piece or two piece; they can drainable or closed; they can be covered or transparent; they may have a filter or no filter; they may have a different type of closure. All the relevant product information and guidance will be given by the stoma care nurse when they leave hospital which can then be built upon as their confidence grows. Overloading someone with too much knowledge about stoma products so soon after surgery can be overwhelming.
Recovery
How long will they be in hospital?
Length of hospital stay will depend upon many factors: the reason for surgery, the surgery itself and their recovery are many factors. One of the most important things is ensuring that their stoma is working after surgery, they are eating and drinking (albeit perhaps lightly) and they can care for the stoma themselves or have someone who can assist them in their setting.
How quickly will they recover?
Recovery is very individual. Factors that affect recovery not only include the type of surgery they had, their reason for surgery or any unforeseen complications, it can also be about how they are mentally coping with the stoma. On average, physically it may be around 4 weeks after keyhole surgery but 2-3 times longer if they had open surgery. Life with a new stoma can often feel like learning a new skill. They will need time to adjust, learn to accept the stoma, build their confidence and set some achievable goals to help them move forward.
Is someone with a stoma disabled?
Disability is not about whether someone can walk a certain distance or have fully functioning limbs. Whether or not someone feels disabled or classes themself as disabled, in the eyes of the law living with a stoma does class someone as having a disability. They are therefore covered under the disability provisions of the Equality Act 2010. The act is there to protect people living with long term chronic illness or a prescribed disability. It does not mean they would be entitled to everything that someone else who is classed as having a disability would. Disability is as much about ability and how their ability is assessed.
Relationships and Pregnancy
I am in a relationship with someone who is due to have an ileostomy, will sex still be possible?
Absolutely. Ordinarily a stoma itself doesn’t mean that sex is not possible or should be any different from what you would normally enjoy. Depending upon their reason for surgery or any health condition, you may need to make some adjustments, but these can usually be worked through with your partner until a solution is found that works for both of you. It is important that you both feel relaxed, wanted, valued and sexy during times of intimacy and having a stoma does not mean that that cannot be the norm.
Some men may experience erectile dysfunction, again depending on surgery, and if this lasts more than a few weeks once they feel ready after surgery, encourage them to discuss this with their stoma care nurse, surgeon or GP. Sometimes internal bruising or swelling around nerves can lead to temporary issues with obtaining an erection.
Women may experience vaginal dryness or loss of sensitivity post surgery and again they can discuss this with their stoma care nurse, surgeon or GP. These people are professionals and are there to help however embarrassed or awkward someone may feel.
Sometimes a little planning can help you both relax before intimacy such as emptying/ changing the bag beforehand or wearing something that makes them feel good about themself and covers their bag initially until they feel more confident.
Medical advice is that you should never introduce any object into a stoma unless it is under medical supervision.
Can my wife/partner still conceive with a stoma?
Yes. It is completely possible to conceive and experience a healthy pregnancy if you have a stoma. As the baby grows and their abdomen changes shape, they may need to adjust their stoma bag to cater for stoma shape. Understandably they may feel concerned or anxious as to how the stoma will behave as they start to grow.
IA can always put your partner in touch with a trained volunteer who has been through pregnancy with a stoma if that helps. Please contact IA national office.
My partner has a stoma but has become withdrawn when it comes to showing affection and at moments of intimacy. What can I do?
This is perfectly normal if your partner is feeling scared, nervous or self-conscious. Perhaps they feel that you don’t love them or want them with a stoma or are embarrassed to be undressed in front of you. Perhaps they need reassurance that you still see them as the same person, that you still love and want them and want to continue a loving relationship. Sometimes going back to basics can help – the things you used to do to make someone feel special and loved outside of the bedroom so that they start to feel appreciated, wanted or special.
It is important not to rush, to allow them the time that they need and take it slowly. Perhaps returning to intimacy isn’t about having sex but stopping short so you both feel desire for each other. If your partner is self-conscious about the way they look when they undress, nice underwear can help to make them feel good about themselves. While specialist underwear is not necessary, there are companies who make underwear for people with a stoma which is designed to cover the stoma bag.
Travel
I am a Nurse or Healthcare Professional
Resources
How do I arrange for my patient to speak to someone about surgery?
IA has a team of volunteers who have been through surgery themselves and are trained to speak to others. IA’s trained volunteers do not offer medical advice but they are able to empathise, listen and offer guidance to others who are going through or have been through surgery. We ensure that all information remains confidential.
To arrange a visit, you can complete our secure form online or contact IA national office on 0800 0184 724.
How do I request IA Journals for my waiting area?
IA would be happy to send you some journals for your waiting area each quarter. Our journal is full of stories, medical articles and product information to keep people informed about living with an ileostomy or an internal pouch. You can arrange regular copies of the journal to be sent to you by completing our online form, calling us on 0800 0184 724 or emailing [email protected].
What support is available through IA to people undergoing surgery?
IA was formed in 1956 and has over 60 years of experience. As a network of groups spread around the UK and Ireland, IA is well placed to offer support through any of our volunteer groups, our quarterly journal, our wealth of literature or our website/social media presence. IA can also arrange for your patient to speak with someone who has been through surgery and answer their questions based on personal experience. To request a chat with someone, please complete our online secure form and we will be in touch.
What support do you offer stoma care nurses?
IA has been working with stoma care nurses since the role began in the early 1970’s. IA was instrumental in the formation of the role and recognises the importance the stoma care nurses provides. We have a range of literature and posters which can be ordered and sent out to you to pass on to patients or for patient waiting areas.
Alternatively members of your local IA group would be happy to support any local stoma care open days or events that you may be running.
IA is also pleased to work with the University of East Anglia to sponsor study opportunities for nurses wanting to develop their stoma care career and take the Advanced Stoma Care module, subject to qualification. For more information about the course content please visit www.uea.ac.uk and search for ‘Advanced stoma care’.
How do I keep up to date with IA’s products and services?
You can join IA’s mailing list and we can keep you up to date with our products and services. We’d also be happy to send you a copy of our quarterly journal or provide copies for your waiting areas. Visit our dedicated page for more information about resources available to healthcare professionals.
How can I get involved with IA?
IA is always looking for nurse presence on its local group committees to help us offer support to our members and non members. If you have some time to spare, however much you can, and would like to be involved please contact the secretary of your local group or contact IA national office to discuss what support you can offer us.
Do you offer a colouring book for young children?
IA is pleased to offer a colouring book for young children which explains in child friendly language what the digestive system is. Our book follows the progress of two children, Zach and Ellie, who have had surgery themselves and provides an opportunity for children to learn while they colour. It’s also a great resource for parents or grandparents who are themselves undergoing surgery and want to explain and reassure their children/grandchildren as to what is happening to them.
“Living with a stoma doesn’t have to be a barrier to enjoying life”
IA teamed up with My Bag and I to release a documentary about living with a stoma. The documentary, directed by Michael Durban, tackles social issues facing ostomates today such as dating, sex, mental wellbeing and day to day living.
The documentary follows ostomates Elliot and Hari who discuss individually, in an honest and open way, how life with a stoma raised fears around dating with a bag and the pre-conceptions that many new to surgery often develop.
We smash those fears and show people that living with a stoma doesn’t have to be a barrier to enjoying life.